G2 BioPharma Services Inc.
Global Partnership in
Contract Clinical Trial Services

our services
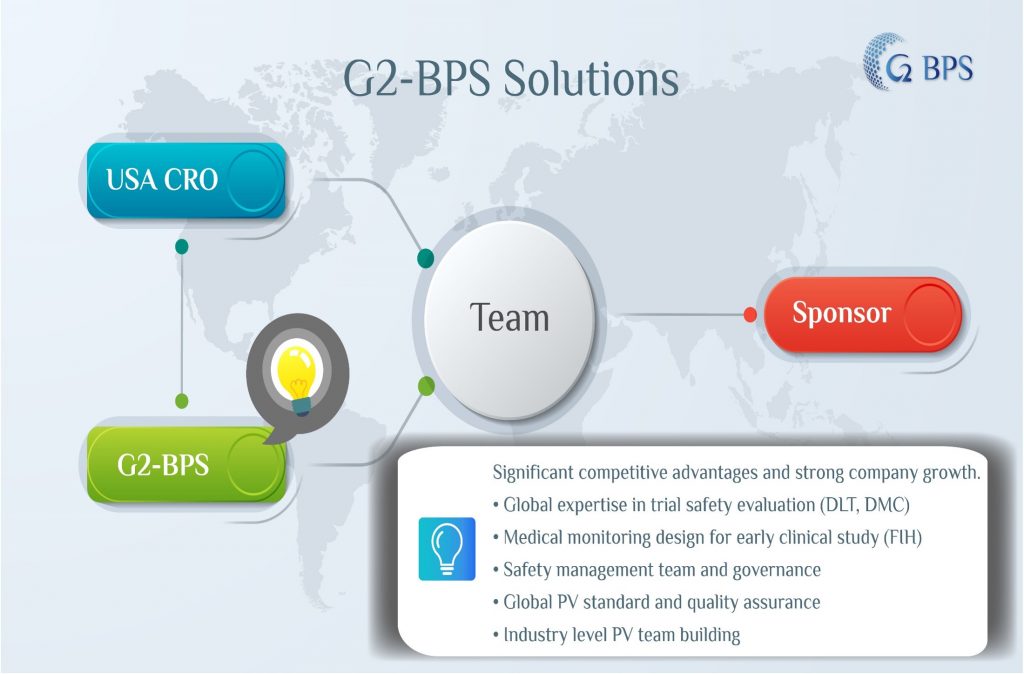
Partner with CRO in Global Clinical Trial Projects

Direct benefits to CRO
Higher chance for Bid success + broader service scope = better industry reputation and increasing company business income

Added benefits
G2-BPS is SME in following critical processes:
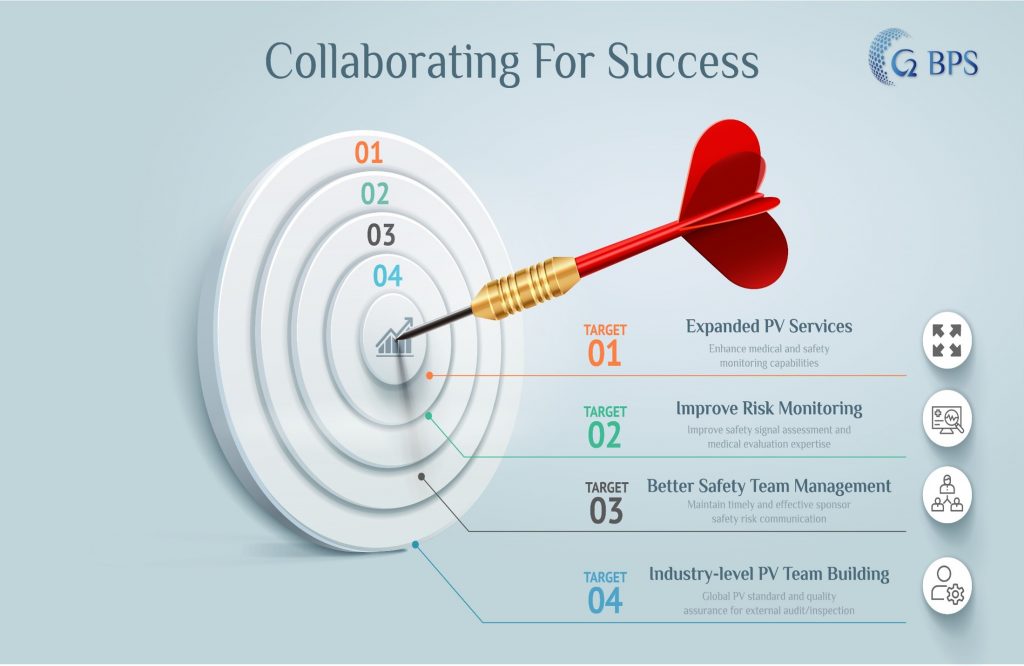
(1)Contribute in CRO’s medical, clinical safety/PV teams building
(2)Help CRO to better prepare client’s business audit inspection
Our packages
Partner with CRO
G2-BPS Performs
the following Medical and Safety Contract Services
G2-BPS Supports
CRO in the Following Medical and Safety Contract Services