G2 BioPharma Services Inc.
Medical Safety Solutions to
FIH Clinical Trials
What Does G2 Offer?
1.Experienced IND application expert team (RA, Medical, Safety, PK/PD, Toxicology, Nonclinical, CMC)
2.Study design communication strategy with HAs (e.g., FDA, PMDA, NMPA)
3.Consultation on critical study documents ( protocol, IB, ICF, MMP, SMP, DSMB Charter, SAP) development
4.24/7 expert services in medical monitoring, safety monitoring, and regulatory reporting (e.g., IND safety report / SUSAR, DSUR)

usability
G2 Services
flexible
G2 Clinical Trials Services
Global or Local Clinical Trials Medical Safety Services
1.Medical monitoring
2.Safety monitoring
3.24/7 SAE/SUSAR processing and medical review
4.Global safety database/tools for safety data processing and submission
5.Periodic aggregate safety data reporting (DSUR)
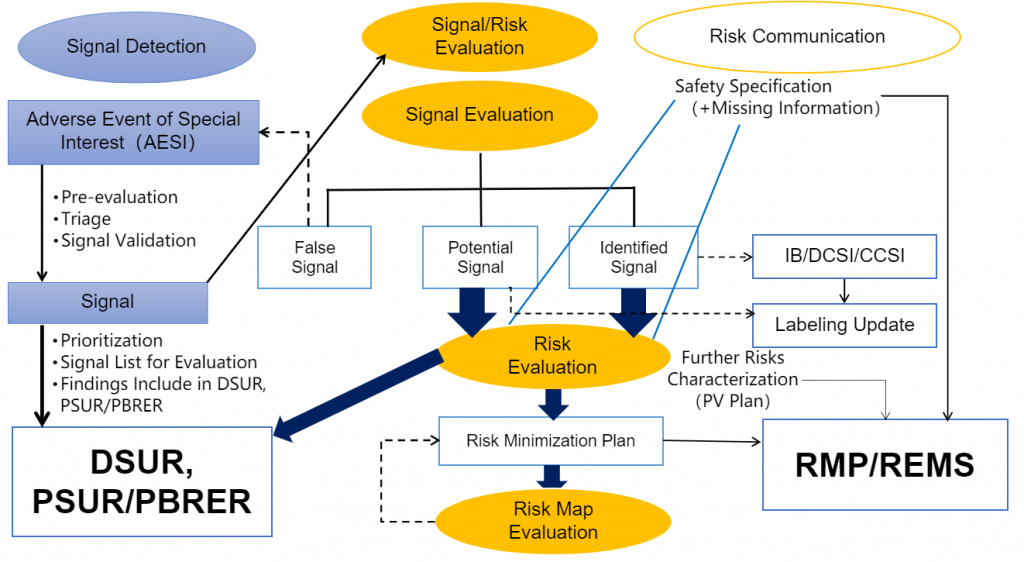
6.New safety signal/AESI detection and risk mitigation strategies
7.SOPs, quality control, trainings, and compliance tracking
8.Site and company external/internal audit and inspection


professional
US FDA IND/NDA Submission and Safety Data Consulting Services
US FDA IND/NDA Submission and Safety Data Consulting Services
1.IND application submission safety monitoring strategy and regulatory communication
2.NDA integrated summary of safety development and submission
3.Global experts/KOLs networking and IRB/EC interaction
4.Trial safety review committee, DSMB/DMC, and interim analysis
important opinion
G2 Expertise
Clinical Trials Medical Monitoring
Safety Signal Detection and Risk Management
Ready to Start?
G2 Expertise Global Expert Consultation
*Integration of pooled safety data for summary and HA submission (IND, NDA, SCS, ISS, CSR)
*Medical safety review of ICSRs (SAEs, SUSARs, US IND safety report & AOSIs, targeted FU)
*Development of aggregate periodic safety data update reports (DSUR, PBRER, PSUR)
*Maintenance of product risk management profile and safety reference documents (RMP, REMS, R/B Assessment, IB/RSI, DCSI, CCSI/CCDS, local labels, e.g., USPI, SmPC, JPI )
*Detection and management of safety signals (data retrieval & management, signal evaluation strategy, data mining & interpretation)
*Medical safety review of published literature (screening, search strategy, findings summary)
*Global safety management team and company safety governance establishment
*Clinical trials safety and post-marketing PV on the job trainings
*Global safety quality system set-up and audits and inspection preparation
*Medical assessment of safety-related product complaint/quality issues/recall/withdrawal
*Development of global safety database, data review tools and applications